algae are important plants, are useful in the soil - some help fix nitrogen or make it bio-available, but of course one of the most interesting uses for algae would be in the production of biofuels. We blogged here about algae being used in reactors (or living foundries) to create a biodiesel, for example.
So what is Algae? Is it just seaweed?
want to know more? here's a 50 minute in-depth seminar/presentation on algae:
but (if you survived that in-depth study) there are continuing developments in farming algae, reported just recently. Recall the algae bio-reactors? Well in this news item here i spied a novel way of locating reactors with minimum land-use and greater efficiency of production - in bags at sea!
If you have been paying even the slightest attention to the algae industry, you probably have heard of companies like Solazyme or Synthetic Genomics, the big names that are making big public strides in the field. Algasol Renewables, on the other hand, is one name in the industry that you have probably never heard mentioned. However, Algasol looks to be on the brink of joining those big names as one of leaders in the algae industry with their photobioreactor system.
Photobioreactors (or PBR’s) come in many different shapes, sizes, and designs. Essentially, they consist of some clear material formed in a way that it can hold an algae-containing liquid. Typically, you will find them looking like long tubes, snaking back and forth, that allow sunlight to reach the algae-water concentration that is pumped through it. They work great for growing algae but have typically suffered from high initial and operating costs.
This is where Algasol comes into play. They have designed a photobioreactor system that can potentially cut costs by 90 percent. How have they done this? Well, their thinking has taken them outside the tube and placed them into a bag.
Basically, their system grows freshwater microalgae in large plastic bags that float on top of bodies of saltwater. There, as in any other bioreactor, nutrients and CO2 are pumped in to feed the algae.
This design led Frost & Sullivan to give Algasol their 2010 “Global Algae Biofuels Award.” According to them, “Algasol Renewables provides a critical and innovative method for micro algae biomass production. Its modular floating bag technology, a new variation of photobioreactors (PBRs), provides a low-cost design coupled with industrial scalability, optimal light exposure, high biomass concentration, low energy consumption, and efficient system control.”
The oceans of the world have a great potential to be the location for floating algae farms. First off, oceans cover around 70 percent of the world. With land (especially agricultural land) becoming a very precious commodity, moving production of fuel offshore is a major bonus.
Additionally, the ocean cuts out a lot of the energy costs associated with traditional PBR’s. For example, the water surrounding the bags acts as a temperature buffer, a process that would require spraying down the outsides of the photobioreactor in typical systems. Also, the wave action in the ocean helps to mix the algae in the bags, something that would otherwise take additional energy in land-based designs.
Now, some may be concerned about putting all this plastic into the ocean should a storm comes along or worried about what happens if these bags break. Luckily, engineers at Algasol have addressed both of these problems. If a storm comes along, the bags have been designed to be submerged beneath the water to levels up to 250 feet. There, they can wait out a tropical storm, hurricane, etc.
Researchers are also not too concerned if one of the bags breaks. Since the algae will be freshwater species, they will die when exposed to saltwater and there, researchers have concluded, they can become food for fish and other marine life.
Anyone who has browsed through the latest research literature is left in no doubt: we should bury the traditional and comforting idea that Homo sapiens is somehow special, separate and "better" than the rest of life on the planet.
A study of remarkable rock formations in western Australia has provided a vivid reminder of our earliest forebears. These 3.4 billion-year-old features of the Pilbara region - some looking like egg cartons, others like crests, waves or upside-down ice-cream cones - are now thought to be the remains of ancient microbial communities that were among the first living things on Earth.
Abigail Allwood of Macquarie University, Sydney, one of the team that studied them, is convinced that these formations - stromatolites - are among the great-grandmothers of all life on our planet. But as DNA mutated and evolved over billions of years, it had been thought that we left our microbial origins far, far behind.
So far, in fact, that most people now regard our ancestors as worse than an embarrassing relative. They perceive bugs as alien, as a threat to our existence. When it comes to bacteria that lurk in hospitals, toilets and kitchens, we seem to be engaged in an endless war. They are germs that must be shown no mercy. They must be destroyed with chemicals, antibacterials and cleaning fluids.
But in the scientific world, there is a growing awareness that we are much more dependent on this "simpler" life than we realise. The best-known proponent of this view is Prof Lynn Margulis of the University of Massachusetts, Amherst, who developed the concept of "endosymbiosis", the idea that our complex cells depend on simpler microbial tenants.
Nature has mixed and matched simpler creatures for aeons. The plants in the window box, trees in the garden and the broccoli at the greengrocer's all date back to ancient ancestors that became verdant only around two billion years ago, when they abducted smaller green creatures that could capture sunbeams and turn them into food. Indeed, Noriko Okamoto and Isao Inouye of the University of Tsukuba, Japan, even discovered a tiny ocean creature on a beach - Hatena ("mysterious" in Japanese) - that seemed to be engaged in a similar process of becoming green.
Prof Margulis believes our senses evolved directly from bacterial ancestors that had found ways to swim toward food and away from noxious gases, or ascend to the well-lit waters at the surface of a pond. Our cells are also powered by the descendants of bacteria that traded chemical energy for a comfortable home, in the guise of structures called mitochondria. These are organelles (which divide up the task of cellular life as organs do for a body) that have their own DNA, passed down from mother to child to drive our muscles, our digestion, and our brains.
As if to underline how mitochondria complicate the human genetic recipe, the genome entrepreneur Dr Craig Venter made an odd discovery when he offered his facilities to identify the remains of those killed in the World Trade Centre on September 11, 2001. His efforts to create the world's biggest forensic laboratory to deal with the crisis had a surprising scientific spin-off: he found that each of us may be a home for more than one mitochondrial genome.
There is also evidence that other ancient unions of cells helped to make us human. Dr Mark Alliegro, of Louisiana State University Health Sciences Centre, and colleagues made a fascinating find when they studied centrosomes, organelles essential to cell division. The centrosome contains RNA, thought by many to be the most ancient genetic material, which helps to translate genes into proteins, among other things.
These structures could multiply independently of their host cells by passing RNA down to the next generation. Could centrosomes, like mitochondria, be the result of some kind of ancient union between the cells of our ancestors and a microbe long ago? "I like to kid around and say centrosomes may be the mother of all latent viral infections," he says.
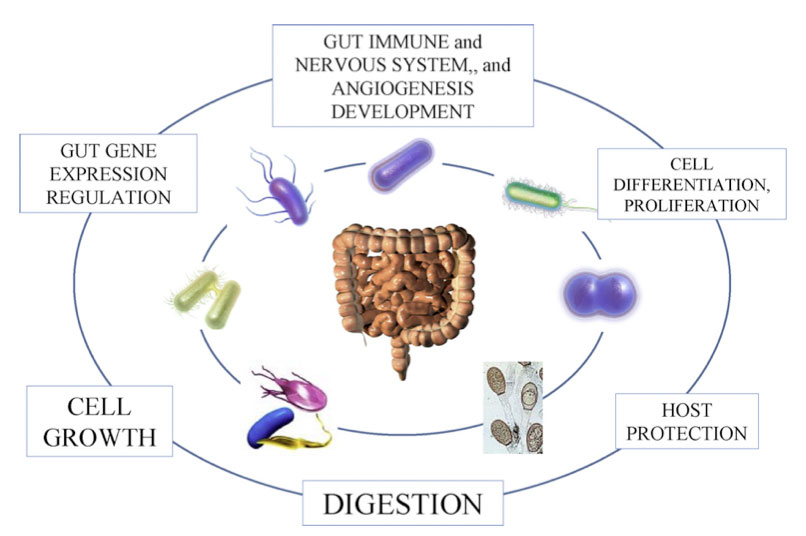
Other passengers in the human super-organism are easier to distinguish from our cells. Scientists have long recognised that the number of human cells in the body is dwarfed by the 100 trillion or so bacteria living in and grazing on it.
This has been obscured by the fact that human cells are much bigger than bacterial cells. As a result, despite their incredible numbers, bacteria account for only about three pounds of the average person's weight.
Just how important those three pounds are, however, has been difficult to weigh up until now. Most bacteria are too fussy to grow in the lab. As a result, little was known about what these majority shareholders really are and what, exactly, they are doing to and for us.
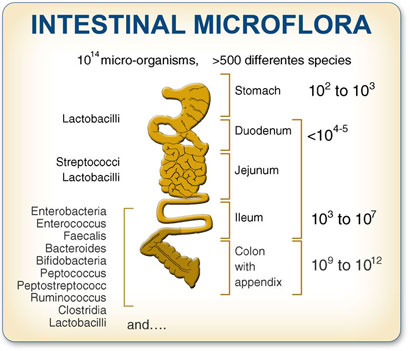
At the Institute for Genomic Research in Maryland, Dr Steven Gill and colleagues decided to investigate the genetic recipe of our bacterial tenants - the "colon microbiome" - by collecting faeces from two anonymous, healthy adults: a man and a woman who had gone without antibiotics or other medications for a year (when faeces is unscathed by antibiotics, half of it is bacteria).
Dr Gill found that we depend on some ancient organisms from what is called the third domain of life. Using DNA screening methods, his team found a surprising number of archaea, also known as archaebacteria, which are genetically distinct from bacteria but are also one-celled organisms often found in extreme environments such as hot springs, or basking in salt and acid.
Overall, they found that the human genome - all the genes in our cells - is but a fraction of what it takes to make a human. The collective bacterial genome in the average person is so large that it contains between 60 and 100 times as many genes as the human genome.
Up to 100 trillion microbes, representing more than 1,000 species, make up a motley "microbiome" that allows humans to digest much of what we eat. We lack the means to break down the food we eat into energy essential for our survival and, while bacteria could survive perfectly well without us, we would be doomed without the toil of bacteria that graze in our guts.
"The GI tract has the most abundant, diverse population of bacteria in the human body," says Dr Gill, now at the State University of New York at Buffalo. "We're entirely dependent on this microbial population for our wellbeing. A shift within this population, often leading to the absence or presence of beneficial microbes, can trigger defects in metabolism and development of diseases such as inflammatory bowel disease."
Dr Gill suspects the ecology of the human gut is at least as complex as that in soils or seas. It teems with single-celled residents that can make vitamins, such as the B vitamins that we cannot synthesise, and can break down plant sugars, such as xylan and cellobiose (similar to cellulose), which humans could not otherwise digest because we lack the necessary enzymes. Our diet would be limited if we could not: cellobiose, for instance, is a key component of plant cell walls that is found in most edible plants, such as apples and carrots.
Some bacteria in the gut break down chemicals made by plants that could cause cancer or other illnesses if they were not neutralised. Others have the capacity to scavenge hydrogen gas from the gut - a byproduct of digestion that can kill helpful bacteria - and convert it into methane. That makes the intestines a more biologically friendly place, while contributing in sometimes embarrassing and smelly incidents to greenhouse emissions. Our intestinal residents even pay us a kind of rent: bacteria in the gut make generous quantities of an enzyme that facilitates the production of butyryl coenzyme A, a fatty acid that is a favourite food of the cells that line the colon.
In short, these gutsy little helpers keep us alive. You would be nothing without the trillions of microbial minions milling around your large intestine, performing crucial physiological functions that your fancy, complicated human cells wouldn't have a clue how to do. These fabulous bugs are part of our inheritance: babies acquire their gut flora as they pass down the birth canal and take a gulp of their mother's vaginal and faecal flora. It might not be the tastiest of first meals, but it could well be one of the healthiest.
This realisation that we are super-organisms gives real meaning to the hazy idea of holistic medicine. Awareness that we depend on "good" bacteria is increasingly being exploited by manufacturers of yogurts and "probiotic" dietary supplements. Soon, doctors will test for changes in the numbers and kinds of microbes in our guts as early indicators of disease. They may prescribe live bacterial supplements to bring certain physiological measures back into normal range. And drug companies will seek compounds that mimic or amplify the actions of beneficial bacteria.
In the rust-red Pilbara desert of Western Australia, an international team of NASA and university researchers looks at ancient rocks to see if they offer unambiguous evidence of life on Earth as long ago as 3.5 billion years. Martin van Kranendonk, and Abby Allwood, show shapes they believe could only have been formed by living organisms. Others on the scientific field trip continue to be skeptical. The ongoing debate shows contemporary research as an exciting intellectual adventure, and looking for life in the Pilbara as a physical challenge. Forget to drink often, says Kranendonk, and you could be dead in less than a day! Life, everywhere on Earth, needs water to survive.
But in the scientific world, there is a growing awareness that we are much more dependent on this "simpler" life than we realise. The best-known proponent of this view is Prof Lynn Margulis of the University of Massachusetts, Amherst, who developed the concept of "endosymbiosis", the idea that our complex cells depend on simpler microbial tenants.
Nature has mixed and matched simpler creatures for aeons. The plants in the window box, trees in the garden and the broccoli at the greengrocer's all date back to ancient ancestors that became verdant only around two billion years ago, when they abducted smaller green creatures that could capture sunbeams and turn them into food. Indeed, Noriko Okamoto and Isao Inouye of the University of Tsukuba, Japan, even discovered a tiny ocean creature on a beach - Hatena ("mysterious" in Japanese) - that seemed to be engaged in a similar process of becoming green.
Prof Margulis believes our senses evolved directly from bacterial ancestors that had found ways to swim toward food and away from noxious gases, or ascend to the well-lit waters at the surface of a pond. Our cells are also powered by the descendants of bacteria that traded chemical energy for a comfortable home, in the guise of structures called mitochondria. These are organelles (which divide up the task of cellular life as organs do for a body) that have their own DNA, passed down from mother to child to drive our muscles, our digestion, and our brains.
As if to underline how mitochondria complicate the human genetic recipe, the genome entrepreneur Dr Craig Venter made an odd discovery when he offered his facilities to identify the remains of those killed in the World Trade Centre on September 11, 2001. His efforts to create the world's biggest forensic laboratory to deal with the crisis had a surprising scientific spin-off: he found that each of us may be a home for more than one mitochondrial genome.
There is also evidence that other ancient unions of cells helped to make us human. Dr Mark Alliegro, of Louisiana State University Health Sciences Centre, and colleagues made a fascinating find when they studied centrosomes, organelles essential to cell division. The centrosome contains RNA, thought by many to be the most ancient genetic material, which helps to translate genes into proteins, among other things.
These structures could multiply independently of their host cells by passing RNA down to the next generation. Could centrosomes, like mitochondria, be the result of some kind of ancient union between the cells of our ancestors and a microbe long ago? "I like to kid around and say centrosomes may be the mother of all latent viral infections," he says.
Other passengers in the human super-organism are easier to distinguish from our cells. Scientists have long recognised that the number of human cells in the body is dwarfed by the 100 trillion or so bacteria living in and grazing on it.
This has been obscured by the fact that human cells are much bigger than bacterial cells. As a result, despite their incredible numbers, bacteria account for only about three pounds of the average person's weight.
Just how important those three pounds are, however, has been difficult to weigh up until now. Most bacteria are too fussy to grow in the lab. As a result, little was known about what these majority shareholders really are and what, exactly, they are doing to and for us.
At the Institute for Genomic Research in Maryland, Dr Steven Gill and colleagues decided to investigate the genetic recipe of our bacterial tenants - the "colon microbiome" - by collecting faeces from two anonymous, healthy adults: a man and a woman who had gone without antibiotics or other medications for a year (when faeces is unscathed by antibiotics, half of it is bacteria).
Dr Gill found that we depend on some ancient organisms from what is called the third domain of life. Using DNA screening methods, his team found a surprising number of archaea, also known as archaebacteria, which are genetically distinct from bacteria but are also one-celled organisms often found in extreme environments such as hot springs, or basking in salt and acid.
Overall, they found that the human genome - all the genes in our cells - is but a fraction of what it takes to make a human. The collective bacterial genome in the average person is so large that it contains between 60 and 100 times as many genes as the human genome.
Up to 100 trillion microbes, representing more than 1,000 species, make up a motley "microbiome" that allows humans to digest much of what we eat. We lack the means to break down the food we eat into energy essential for our survival and, while bacteria could survive perfectly well without us, we would be doomed without the toil of bacteria that graze in our guts.
"The GI tract has the most abundant, diverse population of bacteria in the human body," says Dr Gill, now at the State University of New York at Buffalo. "We're entirely dependent on this microbial population for our wellbeing. A shift within this population, often leading to the absence or presence of beneficial microbes, can trigger defects in metabolism and development of diseases such as inflammatory bowel disease."
Dr Gill suspects the ecology of the human gut is at least as complex as that in soils or seas. It teems with single-celled residents that can make vitamins, such as the B vitamins that we cannot synthesise, and can break down plant sugars, such as xylan and cellobiose (similar to cellulose), which humans could not otherwise digest because we lack the necessary enzymes. Our diet would be limited if we could not: cellobiose, for instance, is a key component of plant cell walls that is found in most edible plants, such as apples and carrots.
Some bacteria in the gut break down chemicals made by plants that could cause cancer or other illnesses if they were not neutralised. Others have the capacity to scavenge hydrogen gas from the gut - a byproduct of digestion that can kill helpful bacteria - and convert it into methane. That makes the intestines a more biologically friendly place, while contributing in sometimes embarrassing and smelly incidents to greenhouse emissions. Our intestinal residents even pay us a kind of rent: bacteria in the gut make generous quantities of an enzyme that facilitates the production of butyryl coenzyme A, a fatty acid that is a favourite food of the cells that line the colon.
In short, these gutsy little helpers keep us alive. You would be nothing without the trillions of microbial minions milling around your large intestine, performing crucial physiological functions that your fancy, complicated human cells wouldn't have a clue how to do. These fabulous bugs are part of our inheritance: babies acquire their gut flora as they pass down the birth canal and take a gulp of their mother's vaginal and faecal flora. It might not be the tastiest of first meals, but it could well be one of the healthiest.
This realisation that we are super-organisms gives real meaning to the hazy idea of holistic medicine. Awareness that we depend on "good" bacteria is increasingly being exploited by manufacturers of yogurts and "probiotic" dietary supplements. Soon, doctors will test for changes in the numbers and kinds of microbes in our guts as early indicators of disease. They may prescribe live bacterial supplements to bring certain physiological measures back into normal range. And drug companies will seek compounds that mimic or amplify the actions of beneficial bacteria.
The message of the latest research is clear: we must learn to love bacteria - they are our ancestors, our tenants and our saviours.
In the rust-red Pilbara desert of Western Australia, an international team of NASA and university researchers looks at ancient rocks to see if they offer unambiguous evidence of life on Earth as long ago as 3.5 billion years. Martin van Kranendonk, and Abby Allwood, show shapes they believe could only have been formed by living organisms. Others on the scientific field trip continue to be skeptical. The ongoing debate shows contemporary research as an exciting intellectual adventure, and looking for life in the Pilbara as a physical challenge. Forget to drink often, says Kranendonk, and you could be dead in less than a day! Life, everywhere on Earth, needs water to survive.